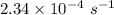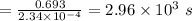Chemistry, 21.09.2019 05:30 lovely8458
The isomerization of methylisonitrile to acetonitrilech3nc(g)→ch3cn(g)is first order in ch3nc . the rate constant for the reaction is 2.34×10−4 s−1 at 489 k .the half-life of the reaction when the initial {\rm [ch_3nc]} is 0.030 m is {\rm s}.a. 1.43×105b. 3.37×10−4c. 4.28×103d. 2.14×103e. 2.96×103

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1
You know the right answer?
The isomerization of methylisonitrile to acetonitrilech3nc(g)→ch3cn(g)is first order in ch3nc . the...
Questions



Mathematics, 04.05.2021 18:40

Mathematics, 04.05.2021 18:40

History, 04.05.2021 18:40





English, 04.05.2021 18:40

English, 04.05.2021 18:40




Mathematics, 04.05.2021 18:40

Chemistry, 04.05.2021 18:40

Social Studies, 04.05.2021 18:40



Health, 04.05.2021 18:40

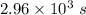
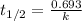
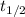 = Half life
= Half life