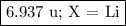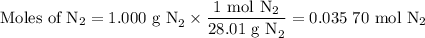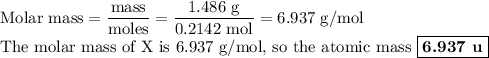Chemistry, 21.09.2019 07:30 MadiAbbott798
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each atom of nitrogen. if 1.486
g of the metal reacts with 1.000 g of nitrogen, what is the calculated atomic mass of the metal?
use your calculated atomic mass to identify the metal. (for your answer, input the proper chemical symbol for element x.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

You know the right answer?
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each...
Questions

Mathematics, 04.03.2020 04:22

History, 04.03.2020 04:22



Physics, 04.03.2020 04:22

Mathematics, 04.03.2020 04:22


Chemistry, 04.03.2020 04:22



Physics, 04.03.2020 04:22

Mathematics, 04.03.2020 04:22

Mathematics, 04.03.2020 04:22




English, 04.03.2020 04:22

Physics, 04.03.2020 04:22


Mathematics, 04.03.2020 04:22