
Chemistry, 21.09.2019 23:10 ayoismeisalex
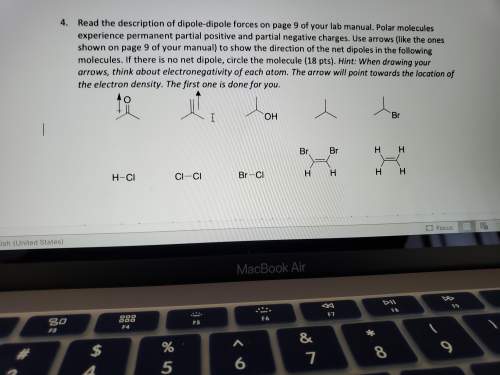
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experience permanent partial positive and partial negative charges. use arrows (like the ones shown on page 9 of your manual) to show the direction of the net dipoles in the following molecules. if there is no net dipole, circle the molecule (18 pts). hint: when drawing your arrows, think about electronegativity of each atom. the arrow will point towards the location of the electron density. the first one is done for you.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experienc...
Questions


Arts, 05.03.2021 15:30

Biology, 05.03.2021 15:30



Geography, 05.03.2021 15:30

English, 05.03.2021 15:30

Mathematics, 05.03.2021 15:30


Spanish, 05.03.2021 15:30





Mathematics, 05.03.2021 15:30


Mathematics, 05.03.2021 15:30


Biology, 05.03.2021 15:30

Chemistry, 05.03.2021 15:30



