
Chemistry, 22.09.2019 03:20 chelseayazzie16
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved to make a final volume of 15 ml of solution. what is the mass/volume percent concentration of this solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved...
Questions


English, 06.10.2019 22:00






Advanced Placement (AP), 06.10.2019 22:00

Social Studies, 06.10.2019 22:00

Mathematics, 06.10.2019 22:00





Mathematics, 06.10.2019 22:00


Social Studies, 06.10.2019 22:00

Computers and Technology, 06.10.2019 22:00

Social Studies, 06.10.2019 22:00

Mathematics, 06.10.2019 22:00

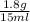 x 100 ml = 12 % m/v
x 100 ml = 12 % m/v

