

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Balance the following ionic equation for a redox reaction, using whole number coefficients.
mn...
mn...
Questions









English, 18.01.2021 21:50







Chemistry, 18.01.2021 21:50



Computers and Technology, 18.01.2021 21:50

History, 18.01.2021 21:50

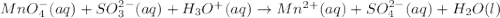
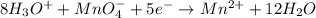 ....... (1)
....... (1)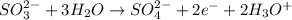 ............. (2)
............. (2)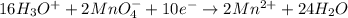 ......... (3)
......... (3)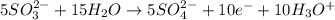 ......... (4)
......... (4)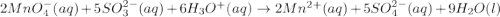
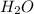 is 9.
is 9.

