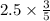Chemistry, 22.09.2019 04:30 sanociahnoel
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o2 (g) - 3co2 (s) + 4h20 (8) when 2.5 mol of o2 are consumed in their reaction, a) 3.0 b) 7.5 c) 1.5 mol of co2 are produced. d) 4.2 e) 2.5

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o...
Questions



History, 25.08.2020 02:01


Mathematics, 25.08.2020 02:01

Physics, 25.08.2020 02:01

Mathematics, 25.08.2020 02:01



Mathematics, 25.08.2020 02:01

Mathematics, 25.08.2020 02:01



Mathematics, 25.08.2020 02:01

Chemistry, 25.08.2020 02:01


Mathematics, 25.08.2020 02:01



English, 25.08.2020 02:01