
Chemistry, 22.09.2019 05:30 rosehayden21
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100∘c. calculate kp for the reaction. n2o4(g)⇌2no2(g) 0.0005 atm 0.095 atm express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100...
Questions



Biology, 18.12.2020 22:40


Biology, 18.12.2020 22:40


Mathematics, 18.12.2020 22:40

Mathematics, 18.12.2020 22:40

History, 18.12.2020 22:40




Mathematics, 18.12.2020 22:40


Mathematics, 18.12.2020 22:40




English, 18.12.2020 22:40

Mathematics, 18.12.2020 22:40

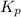 for the reaction is 18.05
for the reaction is 18.05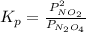
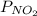 and
and 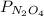 are equilibrium partial pressure of
are equilibrium partial pressure of 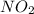 and
and 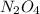 respectively
respectively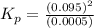 = 18.05
= 18.05

