
Chemistry, 23.09.2019 18:00 townykid96
There are three naturally occurring isotopes of the hypothetical element hilarium 45hi, 46hi, and 48hi. the percentages of these isotopes are the following: hilarium natural isotopes abundance 45hi 18.3% 46hi 34.5% 48hi 47.2% you can assume that the atomic masses are exactly equal to the mass number. calculate the weight of "naturally" occurring hilarium and report it as you would for any other naturally occurring element. answer in units of g/mol. your answer must be within ± 0.005%

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
There are three naturally occurring isotopes of the hypothetical element hilarium 45hi, 46hi, and 48...
Questions

Mathematics, 16.04.2020 08:09

Social Studies, 16.04.2020 08:09


Social Studies, 16.04.2020 08:09

Mathematics, 16.04.2020 08:09

Mathematics, 16.04.2020 08:09


Social Studies, 16.04.2020 08:09

English, 16.04.2020 08:09


Mathematics, 16.04.2020 08:10


Physics, 16.04.2020 08:10


Mathematics, 16.04.2020 08:10




Mathematics, 16.04.2020 08:10

Mathematics, 16.04.2020 08:10

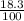 ) + (46 x
) + (46 x 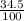 ) + (48 x
) + (48 x 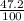 )
)

