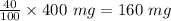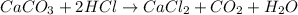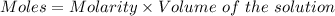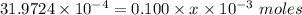Chemistry, 23.09.2019 18:30 GOOBER3838
The antacid component of tumsr is calcium carbonate. assume tumsr is 40.0 percent caco3 by mass. if we have 400. mg of tumsr how many ml of 0.100 m hcl can we neutralize? express your answer in ml.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
The antacid component of tumsr is calcium carbonate. assume tumsr is 40.0 percent caco3 by mass. if...
Questions

Social Studies, 14.10.2021 01:40





Mathematics, 14.10.2021 01:40




Mathematics, 14.10.2021 01:40



Mathematics, 14.10.2021 01:40

Mathematics, 14.10.2021 01:40

Mathematics, 14.10.2021 01:40




Mathematics, 14.10.2021 01:40

History, 14.10.2021 01:40

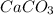 by mass.
by mass.