
Chemistry, 24.09.2019 01:20 RickandMorty420710
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic oxygen, o2. the products and reactants are in a closed container and can all be treated as ideal gases. a. fill in the smallest possible integers that allows the stoichiometry of the reaction equation to be correct: __ kclo3 → kcl o2b. if there are n molecules of potassium chlorate in the initial state, how many product molecules are there

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic...
Questions


Mathematics, 18.01.2021 06:00

Biology, 18.01.2021 06:00

English, 18.01.2021 06:00



Physics, 18.01.2021 06:00


Social Studies, 18.01.2021 06:00




Mathematics, 18.01.2021 06:00

Health, 18.01.2021 06:00

Geography, 18.01.2021 06:10


History, 18.01.2021 06:10



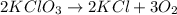
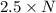 molecules of product.
molecules of product.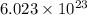 of particles.
of particles.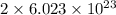 molecules of reactant give
molecules of reactant give 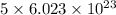 molecules of product
molecules of product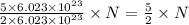 molecules of product.
molecules of product.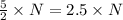 molecules of product are there.
molecules of product are there.

