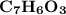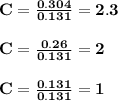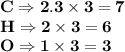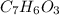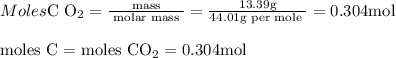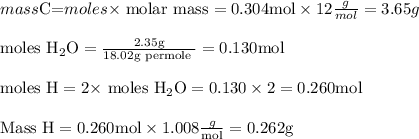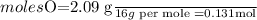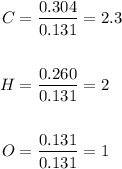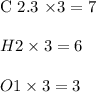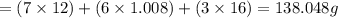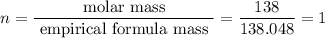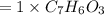Chemistry, 24.09.2019 05:10 Justadumbemo
6.0 g of a certain compound x, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 138 g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
carbon dioxide - 13.39 g
water - 2.35 g
use this information to find the molecular formula of x.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
6.0 g of a certain compound x, known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions

Mathematics, 13.01.2021 23:50



Physics, 13.01.2021 23:50


Chemistry, 13.01.2021 23:50




Mathematics, 13.01.2021 23:50

Health, 13.01.2021 23:50


Chemistry, 13.01.2021 23:50


Computers and Technology, 13.01.2021 23:50



Mathematics, 13.01.2021 23:50