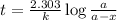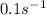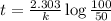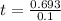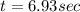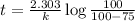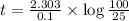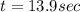Chemistry, 24.09.2019 19:00 robert7248
Assume that you have developed a tracer with first-order kinetics and k = 0.1 s − 1 . for any given mass, how long will it take the tracer concentration to drop by (a) 50% and (b) by 75%?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

You know the right answer?
Assume that you have developed a tracer with first-order kinetics and k = 0.1 s − 1 . for any given...
Questions


Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01




Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Biology, 20.10.2020 04:01


Social Studies, 20.10.2020 04:01

History, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

History, 20.10.2020 04:01