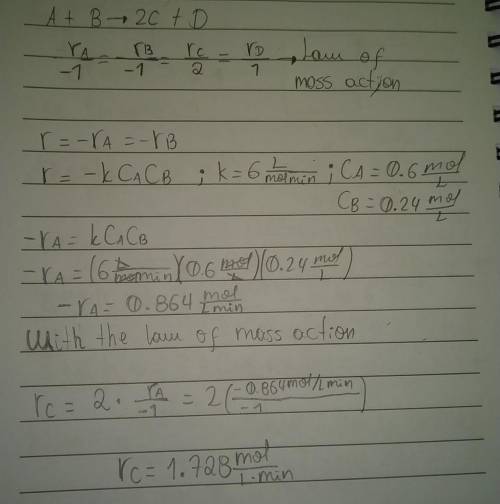Chemistry, 24.09.2019 20:00 soulspiritsa
What is the instantaneous rate of formation of product c given the following information: a. stoichiometric equation a+ b2c+ d b. applicable rate equation is r.-k"ca"cb c. the rate constant is 6.0 liters/(mole-minute) d. the current concentrations of a and b species are ca 0.6 moles/liter and ca 0.24 moles/liter

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2
You know the right answer?
What is the instantaneous rate of formation of product c given the following information: a. stoich...
Questions

History, 07.11.2019 00:31




Mathematics, 07.11.2019 00:31

Mathematics, 07.11.2019 00:31

Physics, 07.11.2019 00:31


Mathematics, 07.11.2019 00:31





Mathematics, 07.11.2019 00:31


Mathematics, 07.11.2019 00:31



English, 07.11.2019 00:31