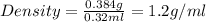Chemistry, 24.09.2019 20:00 prettygirllniyiaa
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chromatography to isolate another common ingredient of headache remedies. the sample of this ingredient had a mass of 384 mg and a volume of 0.32 cm3. looking at the following data, what was the other ingredient in the headache remedy? white table sugar caffeine sodium chloride d -0.70 g/ml d 1.2 g/ml d 2.2 g/ml

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chrom...
Questions



History, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40


History, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40



Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40




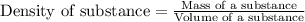
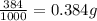 (1g=1000mg)
(1g=1000mg)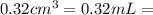 (Conversion factor:
(Conversion factor:  )
)