
Chemistry, 24.09.2019 20:00 AaronMicrosoft15
Amixture of 50 wt% methane, 35 wt% ethane, and 15 wt% propane. determeine the mole fraction of methane.
(can use a basis of 100kg)
b) what is the average molecular weight of the mixture?
for (b) can use m = \sum yi mi where m = average molecular weight, yi= mole fraction of individual substance, mi = molecular weight of individual substance or can also use 1/m = \sum xi / mi where 1/m = average molecular weight, xi = mass fraction of individual substance, mi = molecualr weight of individual substance.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Amixture of 50 wt% methane, 35 wt% ethane, and 15 wt% propane. determeine the mole fraction of metha...
Questions




Computers and Technology, 24.12.2020 16:30



Mathematics, 24.12.2020 16:30



Mathematics, 24.12.2020 16:30





Social Studies, 24.12.2020 16:30

Computers and Technology, 24.12.2020 16:30



English, 24.12.2020 16:30

Mathematics, 24.12.2020 16:30

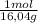 = 31,2 moles
= 31,2 moles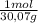 = 11,6 moles
= 11,6 moles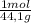 = 3,4 moles
= 3,4 moles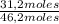 =67,5%
=67,5%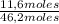 = 25,1%
= 25,1%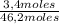 = 7,4%
= 7,4%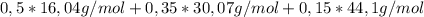 = 25,16g/mol
= 25,16g/mol

