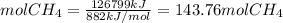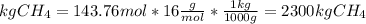The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) and oxygen ( 2 ) is exothermic (-882 kj/mol) with respect to the dissipation of methane. it is of interest to generate 126799 kj of energy from the combustion. how many kg of methane will need to be combusted? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 2.300 o b. 2300.209 o c. 1.725 o d. 0.009

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) a...
Questions


Computers and Technology, 07.06.2021 16:00




Mathematics, 07.06.2021 16:00






English, 07.06.2021 16:00

Mathematics, 07.06.2021 16:00


Mathematics, 07.06.2021 16:00

Arts, 07.06.2021 16:00

Computers and Technology, 07.06.2021 16:00



Social Studies, 07.06.2021 16:00