Chemistry, 24.09.2019 23:20 maskythegamer
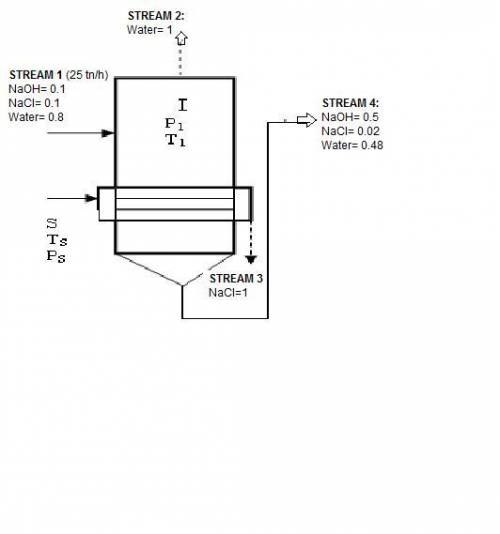
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during evaporation, the water evaporates and the salt precipitates like crystals they are allowed to settle and are removed. the outgoing concentrated solution of the evaporator contains 50% naoh, 2% nacl and 48% water. based on this information is requested:
1. draw the process flow diagram, indicating each of its streams and compositions (known and unknown).
2. calculate the kilograms of precipitated salt and the kilograms of solution concentrated for every hour of work.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1

Chemistry, 23.06.2019 12:00
In ccl4 is there a certain length that the main atom carbon is a certain distance from the chlorine’s?
Answers: 1
You know the right answer?
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during eva...
Questions

Mathematics, 20.10.2019 19:30


Mathematics, 20.10.2019 19:30

Business, 20.10.2019 19:30

Geography, 20.10.2019 19:30

English, 20.10.2019 19:30

Physics, 20.10.2019 19:30



Social Studies, 20.10.2019 19:30

Arts, 20.10.2019 19:30

History, 20.10.2019 19:30

History, 20.10.2019 19:30


History, 20.10.2019 19:30



Chemistry, 20.10.2019 19:30