
Chemistry, 24.09.2019 23:20 Marcynandrew
Amixture of methane and air is capable of being ignited only if the mole percent of methane is between 5% and 15%. a mixture containing 9.0 mole% methane in air flowing at a rate of 700 kg/h is to be diluted with pure air to reduce the methane concentration to the lower flammability limit. calculate the required flow rate of air in mol/h and the percent by mass of oxygen in the product gas. (note: air may be taken to consist of 21 mole% o and 79% n2 and to have an average molecular weight of 29.0.)
(a)n = 20,200 mol air/h; 0.225 kg o2/kg

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Amixture of methane and air is capable of being ignited only if the mole percent of methane is betwe...
Questions










Mathematics, 23.06.2019 23:30




Mathematics, 23.06.2019 23:30


Advanced Placement (AP), 23.06.2019 23:30



Mathematics, 23.06.2019 23:30

Mathematics, 23.06.2019 23:30

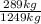 =0,231 kg O₂/ kg air
=0,231 kg O₂/ kg air

