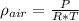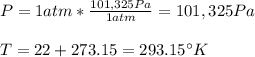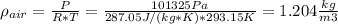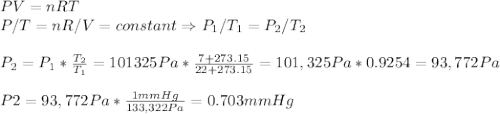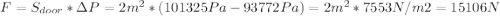Chemistry, 24.09.2019 23:30 imagodatfortnite
An air tight freezer measures 4 mx 5 m x 2.5 m high. with the door open, it fills with 22 °c air at 1 atm pressure.
a. calculate the density of this air in kg/m3
b. after closing the door it is cooled down to 7 °c. how low will the pressure in the freezer
be in units of pa and mmhg?
c. how many newtons of force will be needed to open the 1 m x 2 m door?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
An air tight freezer measures 4 mx 5 m x 2.5 m high. with the door open, it fills with 22 °c air at...
Questions


Mathematics, 10.10.2020 01:01



Health, 10.10.2020 01:01





Biology, 10.10.2020 01:01





History, 10.10.2020 01:01