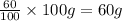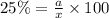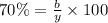Chemistry, 25.09.2019 00:10 19wawrzkeek
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that are in the liquid and solid phases at a given temperature, if the total mass of alloy is 100 g, component b represents 60 % of the alloy, 25 % of the liquid is component b, and 70% of solid is component b.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that a...
Questions

Mathematics, 24.08.2021 02:40


Mathematics, 24.08.2021 02:40


Mathematics, 24.08.2021 02:40





Chemistry, 24.08.2021 02:40

Mathematics, 24.08.2021 02:40

Biology, 24.08.2021 02:40

Mathematics, 24.08.2021 02:40


Social Studies, 24.08.2021 02:40

Social Studies, 24.08.2021 02:40


Arts, 24.08.2021 02:40


Biology, 24.08.2021 02:40