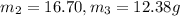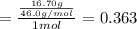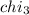Chemistry, 25.09.2019 00:20 latdoz0952
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw = 32.0). using two different analytical techniques to analyze the mixture, it was determined that the water mole fraction was 0.250 while the water mass fraction was 0.134. determine the mole fraction ethanol (c2h5oh) and the mole fraction methanol (ch3oh) in the solution. report the values to the correct number of significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3
You know the right answer?
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw...
Questions

Mathematics, 26.02.2022 06:30


Mathematics, 26.02.2022 06:30

Mathematics, 26.02.2022 06:30



Biology, 26.02.2022 06:40

English, 26.02.2022 06:40


Biology, 26.02.2022 06:40

Physics, 26.02.2022 06:40




Mathematics, 26.02.2022 06:40

Mathematics, 26.02.2022 06:40

Mathematics, 26.02.2022 06:40



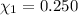
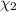
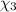
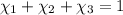
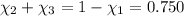
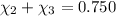
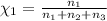
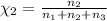
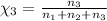
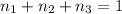
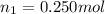
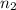
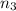
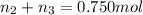
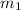
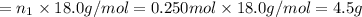
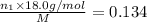
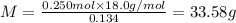
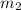
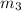
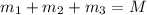
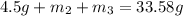
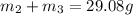 ..[1]
..[1]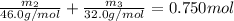
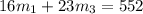 ..[2]
..[2]