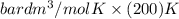Chemistry, 25.09.2019 01:00 nene3210204
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equation of state. the van der waals parameters a and b for ar are 1.355 bar dm mol-2 and 0.0320 dm3mol? , respectively. write your answer (unit: bar) with 2 decimals, as 12.23. do not add unit to your answer.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3


Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equa...
Questions


Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01


Engineering, 26.08.2020 06:01

Biology, 26.08.2020 06:01




Physics, 26.08.2020 06:01

Spanish, 26.08.2020 06:01

Social Studies, 26.08.2020 06:01

History, 26.08.2020 06:01


Mathematics, 26.08.2020 06:01


Mathematics, 26.08.2020 06:01


Mathematics, 26.08.2020 06:01

Mathematics, 26.08.2020 06:01

 = RT
= RT  = v; which is called molar volume
= v; which is called molar volume = RT
= RT
 = 0.08314
= 0.08314