
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is produced. assuming that the reaction proceeds to completion, determine the composition of the exit gas stream both in mole and mass %. if 75% of nitrogen gets converted, what will be the corresponding fractions?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3
You know the right answer?
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is prod...
Questions


Arts, 28.01.2021 19:20



Mathematics, 28.01.2021 19:20

Mathematics, 28.01.2021 19:20





English, 28.01.2021 19:20



English, 28.01.2021 19:20


Mathematics, 28.01.2021 19:20


Mathematics, 28.01.2021 19:20


Mathematics, 28.01.2021 19:20

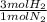 = 60 moles of H₂(g)
= 60 moles of H₂(g) 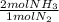 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)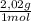 = 20,2 g of H₂
= 20,2 g of H₂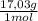 = 681,2 g of NH₃
= 681,2 g of NH₃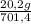 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂; 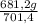 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃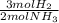 = 45 moles of H₂(g)
= 45 moles of H₂(g) 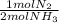 = 15 moles of N₂(g)
= 15 moles of N₂(g) 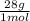 = 140 g of N₂
= 140 g of N₂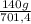 ₓ 100 =20% N₂;
ₓ 100 =20% N₂; 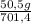 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂; 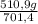 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃

