
Chemistry, 25.09.2019 01:10 cocacola8268
Radioactive decay can be described by the following equation in a = in ao- kt where ao is the original amount of the substance, a is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. minutes for the radioactive isotope chromium-56, k is 1.17 x 10 if the original amount of chromium-56 in a sample is 34.5 mg, how much time is needed for the amount of chromium- 56 that remains to fall to 20.3 mg?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2


Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1
You know the right answer?
Radioactive decay can be described by the following equation in a = in ao- kt where ao is the origin...
Questions



History, 21.06.2019 21:20


Mathematics, 21.06.2019 21:20


History, 21.06.2019 21:20




Mathematics, 21.06.2019 21:20



Mathematics, 21.06.2019 21:20

English, 21.06.2019 21:20





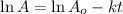
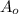 = initial mass of Cr-56 isotope = 34.5 mg
= initial mass of Cr-56 isotope = 34.5 mg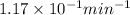
![\ln (20.3)=\ln (34.5)-[(1.17\times 10^{-1}min^{-1})\times t]}\\\\t=\frac{\ln(34.5)-\ln(20.3)}{1.17\times 10^{-1}}=4.53min](/tpl/images/0259/8505/00b1f.png)


