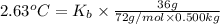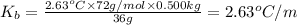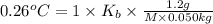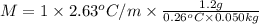The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene increases the boiling point of the solution to 82.73ºc
a. consider the benzene boiling point constant. show calculations.
b. in dissolving 1.2 g of unknown solute in 50 g of benzene, a solution with a boiling point of 80.36ºc is obtained, which is the molar mass of the solute (assume that i = 1) (show calculations)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene inc...
Questions

Mathematics, 23.10.2020 08:01

Mathematics, 23.10.2020 08:01

Mathematics, 23.10.2020 08:01







Mathematics, 23.10.2020 08:01




Mathematics, 23.10.2020 08:01

Biology, 23.10.2020 08:01


English, 23.10.2020 08:01

Social Studies, 23.10.2020 08:01

History, 23.10.2020 08:01

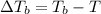
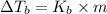
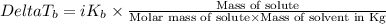
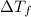 =Elevation in boiling point
=Elevation in boiling point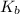 = boiling point constant od solvent= 3.63 °C/m
= boiling point constant od solvent= 3.63 °C/m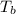 =82.73°C
=82.73°C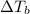 = 82.73°C - 80.1°C = 2.63°C
= 82.73°C - 80.1°C = 2.63°C