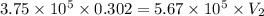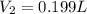Chemistry, 25.09.2019 01:20 JimmySample7
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a temperature of 23.6°c. what volume of gas has been released into the atmosphere if the final pressure in the cylinder is 5.67 x 105 pa? assume ideal behavior and that the gas temperature is unchanged.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a tempera...
Questions

Mathematics, 16.09.2021 19:00


Physics, 16.09.2021 19:00




Social Studies, 16.09.2021 19:00


Mathematics, 16.09.2021 19:00

Social Studies, 16.09.2021 19:00

Mathematics, 16.09.2021 19:00



Computers and Technology, 16.09.2021 19:00

Biology, 16.09.2021 19:00

Geography, 16.09.2021 19:00


Computers and Technology, 16.09.2021 19:00


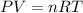
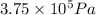 = 3675 atm (1 kPa= 0.0098 atm)
= 3675 atm (1 kPa= 0.0098 atm)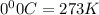
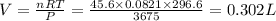
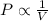 (At constant temperature and number of moles)
(At constant temperature and number of moles)
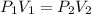
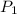 = initial pressure of gas =
= initial pressure of gas = 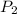 = final pressure of gas =
= final pressure of gas = 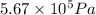
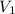 = initial volume of gas = 0.302 L
= initial volume of gas = 0.302 L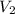 = final volume of gas = ?
= final volume of gas = ?