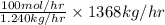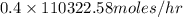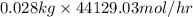Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Pure nitrogen (n2) and pure hydrogen (h2) are fed to a mixer. the product stream has 40.0% mole nitr...
Questions

Mathematics, 03.11.2020 21:50

Computers and Technology, 03.11.2020 21:50




Mathematics, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50




Social Studies, 03.11.2020 21:50



Mathematics, 03.11.2020 21:50

History, 03.11.2020 21:50


English, 03.11.2020 21:50



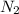 in feed = 40 mole%
in feed = 40 mole%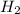 in feed = (100 - 40)% = 60%
in feed = (100 - 40)% = 60%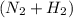 in feed stream.
in feed stream.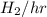 (2 g/mol of
(2 g/mol of 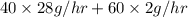
 (as 1 kg = 1000 g)
(as 1 kg = 1000 g)