Chemistry, 25.09.2019 02:00 AriesDaWolf
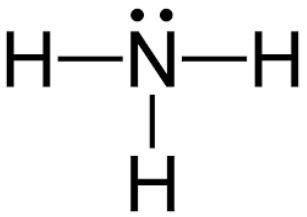
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral because there are four bonded pairs around nitrogen.
it is trigonal pyramidal because there are four bonded pairs around nitrogen.
it is tetrahedral because there are three bonded pairs and one lone pair around nitrogen.
it is trigonal pyramidal because there are three bonded pairs and one lone pair around nitrogen.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral...
it is tetrahedral...
Questions





History, 25.09.2019 21:00



Computers and Technology, 25.09.2019 21:00



Mathematics, 25.09.2019 21:00

History, 25.09.2019 21:00

History, 25.09.2019 21:00


Mathematics, 25.09.2019 21:00

Arts, 25.09.2019 21:00


Mathematics, 25.09.2019 21:00


English, 25.09.2019 21:00