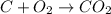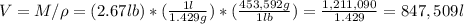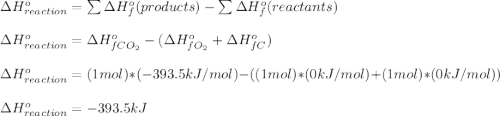Chemistry, 25.09.2019 01:30 kayleighanne3462
Every year people die from carbon monoxide poisoning because they bring a charcoal fire into their tent, house, or enclosed area. when carbon burns, it can produce either carbon dioxide, co2(g), or carbon monoxide gas, co(g).
a) if you start with a system containing 1 pound of charcoal briquettes (assuming pure carbon), how many grams of oxygen is needed to turn all of the carbon into the safe carbon dioxide?
b) what volume of air is required (in l)?
c) how much heat is given off as a result of the combustion to co2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
Every year people die from carbon monoxide poisoning because they bring a charcoal fire into their t...
Questions


Mathematics, 02.09.2020 21:01

History, 02.09.2020 21:01













Health, 02.09.2020 21:01

Biology, 02.09.2020 21:01