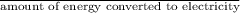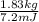Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of the coal is 50% by mass. 4. calculate the co2 production in kg/mj if a coal fired power plant (efficiency - 30%) is used to produce electricity. assume energy density of coal - 24 mj/kg and assume the coal is

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3



Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of...
Questions

History, 25.06.2019 18:30



Chemistry, 25.06.2019 18:30


Biology, 25.06.2019 18:30


History, 25.06.2019 18:30

Chemistry, 25.06.2019 18:30

Spanish, 25.06.2019 18:30

Mathematics, 25.06.2019 18:30

Mathematics, 25.06.2019 18:30

Mathematics, 25.06.2019 18:30



Spanish, 25.06.2019 18:30

Arts, 25.06.2019 18:30

Mathematics, 25.06.2019 18:30

Arts, 25.06.2019 18:30

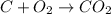
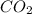 = 44 kg/kmol
= 44 kg/kmol
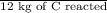
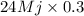 = 7.2 Mj
= 7.2 Mj