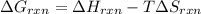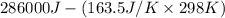Chemistry, 25.09.2019 02:20 destinyhammons12345
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid water at 298°k and 1 atm. the chemical reaction is h2001) h2(g) + 0.502(g) data (at 298°k and 1 atm): ah = 286 kj for this reaction, suzo = 70 jk, sh2 = 131 jik, and soz = 205 j/ºk.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid...
Questions

Biology, 02.03.2021 09:30


Mathematics, 02.03.2021 09:30




History, 02.03.2021 09:30



History, 02.03.2021 09:30


Mathematics, 02.03.2021 09:30

Mathematics, 02.03.2021 09:30

Mathematics, 02.03.2021 09:30


English, 02.03.2021 09:30

Mathematics, 02.03.2021 09:30



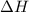 = 286 kJ =
= 286 kJ = 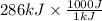
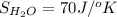 ,
, 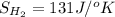
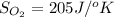
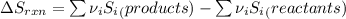
![[(\frac{1}{2} \times S_{O_{2}}) - (1 \times S_{H_{2}})] - [1 \times S_{H_{2}O}]](/tpl/images/0260/0439/5a90c.png)
![[(\frac{1}{2} \times 205) + (1 \times 131)] - [(1 \times 70)]](/tpl/images/0260/0439/31e9d.png)