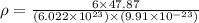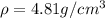Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
Titanium has an hcp unit cell for which the ratio of the lattice parameters cais 1.58. if the radius...
Questions




Computers and Technology, 13.07.2020 21:01






Mathematics, 13.07.2020 21:01

Mathematics, 13.07.2020 21:01




Mathematics, 13.07.2020 21:01

Mathematics, 13.07.2020 21:01



Chemistry, 13.07.2020 21:01

Computers and Technology, 13.07.2020 21:01

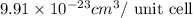
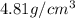
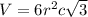
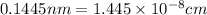
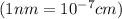
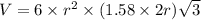
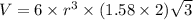
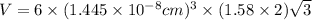
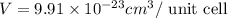
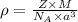
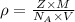 ..........(1)
..........(1)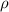 = density of Ti = ?
= density of Ti = ?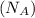 = Avogadro's number =
= Avogadro's number = 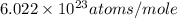
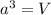 = volume of unit cell =
= volume of unit cell =