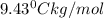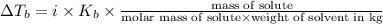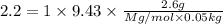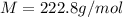A2.60 gram sample of a compound know to contain only indium and chlorine is dissolved in 50.0 g of tin(iv) chloride (kb = 9.43oc kg mol-1). the normal boiling point is raised from 114.1oc for pure sncl4 to 116.3oc for the solution. what is the molecular weight and probable molecular formula of the solute?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
A2.60 gram sample of a compound know to contain only indium and chlorine is dissolved in 50.0 g of t...
Questions

Biology, 24.07.2020 03:01



Mathematics, 24.07.2020 03:01



Mathematics, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01



History, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01


Mathematics, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01

Business, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01

Mathematics, 24.07.2020 03:01



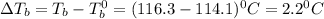 = elevation in boiling point
= elevation in boiling point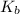 = boiling point constant =
= boiling point constant =