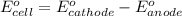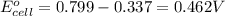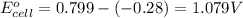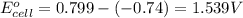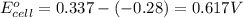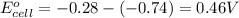Chemistry, 26.09.2019 16:30 savage5447
The standard reduction potentials of the following half-reactions are given in appendix e in the textbook:
ag+(aq)+e−→ag(s)= .799
cu2+(aq)+2e−→cu(s)= .337
ni2+(aq)+2e−→ni(s)= -.28
cr3+(aq)+3e−→cr(s). = -.74
1. determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell emf.
1st and 2nd,
1st and 3rd,
1st and 4th,
2nd and 3rd,
3rd and 4th.
it isn't the first or last one because i have gotten it wrong twice.
2. calculate the value of this emf.
3. then determine which combination is the smallest and calculate the emf.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
The standard reduction potentials of the following half-reactions are given in appendix e in the tex...
Questions


Mathematics, 05.11.2020 19:50

English, 05.11.2020 19:50



Social Studies, 05.11.2020 19:50




History, 05.11.2020 19:50






Social Studies, 05.11.2020 19:50

English, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50


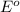 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.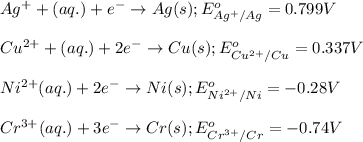
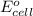 of the reaction, we use the equation:
of the reaction, we use the equation: