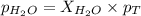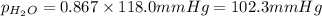Chemistry, 26.09.2019 19:00 Tyrant4life
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoch2ch2oh at 55 °c. the partial pressure of pure water at 55.0 °c is 118.0 mm hg. assume ideal behavior for the solution.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

You know the right answer?
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoc...
Questions




Geography, 03.01.2020 19:31




Computers and Technology, 03.01.2020 19:31






English, 03.01.2020 19:31



Social Studies, 03.01.2020 19:31



Social Studies, 03.01.2020 19:31

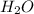 is 102.3 mmHg.
is 102.3 mmHg.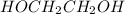 = 33.2 g
= 33.2 g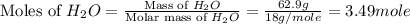
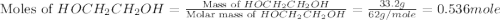
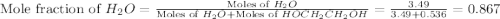
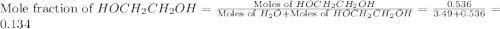
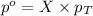
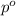 = partial pressure of water vapor
= partial pressure of water vapor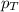 = total pressure of gas
= total pressure of gas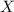 = mole fraction of water vapor
= mole fraction of water vapor