
Chemistry, 26.09.2019 20:00 HighSchool97654
5. the alcohol content of hard liquor is normally given in terms of the "proof," which is defined as twice the percentage by volume of ethanol (ch3oh) present. calculate the number of grams of alcohol present in 1.00 l of 75-proof gin. the density of ethanol is 0.798 g/ml. (4 points)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3
You know the right answer?
5. the alcohol content of hard liquor is normally given in terms of the "proof," which is defined as...
Questions

Chemistry, 31.03.2020 20:36



Mathematics, 31.03.2020 20:36




Mathematics, 31.03.2020 20:36


Mathematics, 31.03.2020 20:36




History, 31.03.2020 20:36

English, 31.03.2020 20:36

English, 31.03.2020 20:37



Mathematics, 31.03.2020 20:37

Health, 31.03.2020 20:37

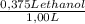
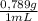 = 299 g of ethanol presents in 1,00 L of 75-proof gin
= 299 g of ethanol presents in 1,00 L of 75-proof gin

