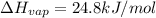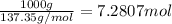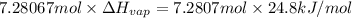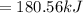Chemistry, 26.09.2019 19:30 Kekkdkskdkdk
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and its enthalpy of vaporization is 24.8 kj/mol at its normal boiling point of 24c. ideally how much energy in the form of heat is removed from a room by an air conditioner that evaporates 1.00 kg of freon-11?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

You know the right answer?
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and...
Questions




Biology, 04.07.2019 15:40




History, 04.07.2019 15:40


Spanish, 04.07.2019 15:40


Mathematics, 04.07.2019 15:40





Mathematics, 04.07.2019 15:40


History, 04.07.2019 15:40

Health, 04.07.2019 15:40