Chemistry, 26.09.2019 21:30 dayanaraa61
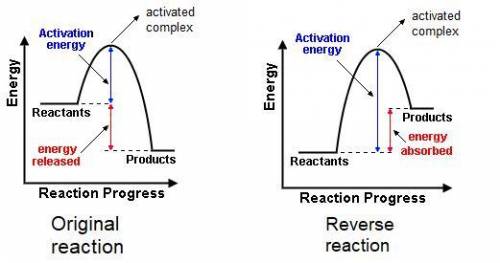
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the activation energy for the reaction is 7 kj. a) draw the potential energy curve for the reaction and label ea, ∆e, and the activated complex or transition state. b) draw the potential curve for the reverse reaction. what is ea? what is ∆e?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the...
Questions






Mathematics, 11.03.2021 02:10

Mathematics, 11.03.2021 02:10

Mathematics, 11.03.2021 02:10



Mathematics, 11.03.2021 02:10

Computers and Technology, 11.03.2021 02:10

Mathematics, 11.03.2021 02:10

Mathematics, 11.03.2021 02:10


English, 11.03.2021 02:10

History, 11.03.2021 02:10


Social Studies, 11.03.2021 02:10

Mathematics, 11.03.2021 02:10