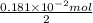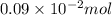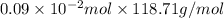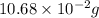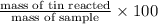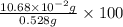Chemistry, 26.09.2019 22:20 dramaqueenactr2040
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents. a sample of impure tin of mass 0.528 g is dissolved in strong acid to give a solution of sn2+. the solution is then titrated with a 0.0448 m solution of no3−, which is reduced to no(g). the equivalence point is reached upon the addition of 4.03×10−2 l of the no3− solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing...
Questions




Mathematics, 29.07.2021 06:40



Mathematics, 29.07.2021 06:40

Mathematics, 29.07.2021 06:40


English, 29.07.2021 06:50

Mathematics, 29.07.2021 06:50

Mathematics, 29.07.2021 06:50



Advanced Placement (AP), 29.07.2021 06:50

History, 29.07.2021 06:50


Mathematics, 29.07.2021 06:50


Mathematics, 29.07.2021 06:50

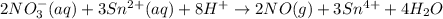
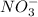 consumed will be calculated as follows.
consumed will be calculated as follows.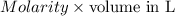
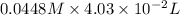
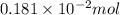
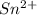 .
.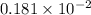 moles of
moles of