
Chemistry, 27.09.2019 01:10 maybeemmamay
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lithium nitride is g. molar mass of li is 6.94 g/mol. molar mass of n2 is 28.02 g/mol. molar mass of li3n is 34.83 g/mol.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lit...
Questions

Mathematics, 08.10.2019 10:00

Mathematics, 08.10.2019 10:00


Mathematics, 08.10.2019 10:00

Chemistry, 08.10.2019 10:00



Biology, 08.10.2019 10:00


Mathematics, 08.10.2019 10:00

Mathematics, 08.10.2019 10:00

History, 08.10.2019 10:00

Computers and Technology, 08.10.2019 10:00

Biology, 08.10.2019 10:00

Mathematics, 08.10.2019 10:00

Biology, 08.10.2019 10:00


Biology, 08.10.2019 10:00

Mathematics, 08.10.2019 10:00

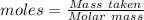
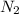
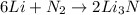
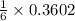 mole of
mole of 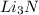
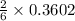 mole of
mole of 

