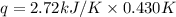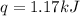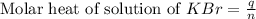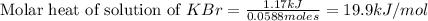Chemistry, 27.09.2019 02:30 bracefacer42
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat capacity of 2.72 kj⋅k−1,2.72 kj⋅k−1, the temperature decreases by 0.430 k.0.430 k. calculate the molar heat of solution of kbr.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat ca...
Questions

Medicine, 24.04.2021 23:40

Mathematics, 24.04.2021 23:40

Mathematics, 24.04.2021 23:40

Chemistry, 24.04.2021 23:40

History, 24.04.2021 23:40



Engineering, 24.04.2021 23:40



History, 24.04.2021 23:40

Mathematics, 24.04.2021 23:40

Arts, 24.04.2021 23:40

Mathematics, 24.04.2021 23:40

Arts, 24.04.2021 23:40




English, 24.04.2021 23:40

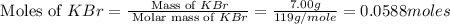
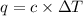
 = heat capacity =
= heat capacity = 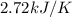
 = change in temperature = 0.430 K
= change in temperature = 0.430 K