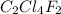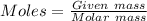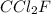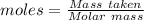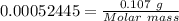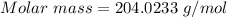Chemistry, 27.09.2019 02:30 tateandvioletAHS14AY
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in another experiment, you find that 0.107 g of the compound fills a 458-ml flask at 25 °c with a pressure of 21.3 mm hg. what is the molecular formula of the compound?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2

Chemistry, 23.06.2019 14:30
Among the elements of the main group the first ionization energy increases
Answers: 3
You know the right answer?
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in...
Questions

Mathematics, 19.09.2019 05:30


History, 19.09.2019 05:30


Biology, 19.09.2019 05:30



Business, 19.09.2019 05:30



Social Studies, 19.09.2019 05:30


Social Studies, 19.09.2019 05:30




Physics, 19.09.2019 05:30

English, 19.09.2019 05:30