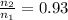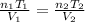Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is then heated to 58°c, causing the balloon to expand to a volume of . what is the ratio of the number of moles of air in the heated balloon to the original number of moles of air in the balloon? (hint: openings in the balloon allow air to flow in and out. thus the pressure in the balloon is always the same as that of the atmosphere.) ratio = 1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is...
Questions



Mathematics, 04.06.2021 23:20


Mathematics, 04.06.2021 23:20

English, 04.06.2021 23:20


Mathematics, 04.06.2021 23:20


Mathematics, 04.06.2021 23:20

Mathematics, 04.06.2021 23:20

Mathematics, 04.06.2021 23:20

Mathematics, 04.06.2021 23:20

Mathematics, 04.06.2021 23:20

History, 04.06.2021 23:20


Mathematics, 04.06.2021 23:20


Mathematics, 04.06.2021 23:20