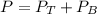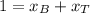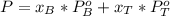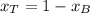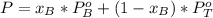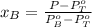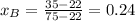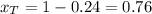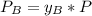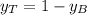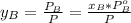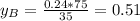Chemistry, 27.09.2019 04:10 chamarabrown9260
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pressure of 35 torr at 20 °c? assume the mixture formsan ideal solution. the vapor pressure of benzene (c6h6) is 75 torr and the vapor pressure of toluene (c7h8)is 22 torr at 20 °c. b) what is the composition in mole fractions of the vapor above the solution in part a? how does this problem relate to the process of fractional distillation?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pr...
Questions





English, 17.07.2019 08:30

Physics, 17.07.2019 08:30

History, 17.07.2019 08:30

Mathematics, 17.07.2019 08:30



Mathematics, 17.07.2019 08:30

Physics, 17.07.2019 08:30






Health, 17.07.2019 08:30

Mathematics, 17.07.2019 08:30

Mathematics, 17.07.2019 08:30

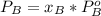
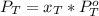
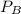 is partial pressure for benzene in the liquid
is partial pressure for benzene in the liquid 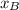 is benzene molar fraction in the liquid
is benzene molar fraction in the liquid 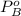 vapor pressure for pure benzene.
vapor pressure for pure benzene.