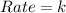Chemistry, 27.09.2019 06:00 kharmaculpepper
Be sure to answer all parts. determine the overall orders of the reactions to which the following rate laws apply: (a) rate = k[no2]2 (b) rate = k zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third ord

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1
You know the right answer?
Be sure to answer all parts. determine the overall orders of the reactions to which the following ra...
Questions

Mathematics, 08.07.2019 00:20


Arts, 08.07.2019 00:20


History, 08.07.2019 00:20


History, 08.07.2019 00:20

Mathematics, 08.07.2019 00:20



French, 08.07.2019 00:20

Mathematics, 08.07.2019 00:20

History, 08.07.2019 00:20




Biology, 08.07.2019 00:20

Mathematics, 08.07.2019 00:20


History, 08.07.2019 00:20

![Rate=k[NO_2]^2](/tpl/images/0267/1831/d48ef.png)
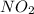 is 2.
is 2.