
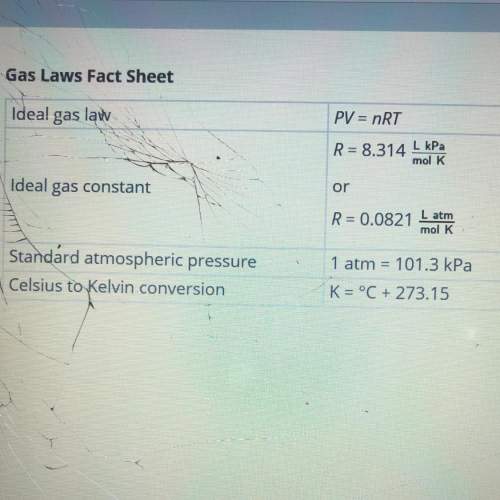
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at temperature of 495c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that t...
Questions


History, 24.07.2019 02:00

Geography, 24.07.2019 02:00

Chemistry, 24.07.2019 02:00


Computers and Technology, 24.07.2019 02:00



Social Studies, 24.07.2019 02:00

Arts, 24.07.2019 02:00


Mathematics, 24.07.2019 02:00



Mathematics, 24.07.2019 02:00

History, 24.07.2019 02:00

History, 24.07.2019 02:00

History, 24.07.2019 02:00

Spanish, 24.07.2019 02:00

Arts, 24.07.2019 02:00



