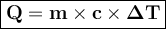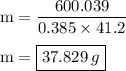Chemistry, 27.09.2019 17:30 Annaborden02
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a beaker containing 95.7 g of water at 22.7 ∘c. when the two substances reach thermal equilibrium, the final temperature is 24.2 ∘c. what is the mass of the copper block? express your answer in grams to three significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a...
Questions

Mathematics, 04.04.2020 16:30

Advanced Placement (AP), 04.04.2020 16:30

Mathematics, 04.04.2020 16:30



Mathematics, 04.04.2020 16:30

Mathematics, 04.04.2020 16:30

Mathematics, 04.04.2020 16:30

Mathematics, 04.04.2020 16:30




Physics, 04.04.2020 16:31

Mathematics, 04.04.2020 16:31

Arts, 04.04.2020 16:31

Mathematics, 04.04.2020 16:31

English, 04.04.2020 16:31

Mathematics, 04.04.2020 16:31

History, 04.04.2020 16:31