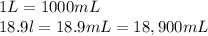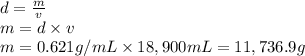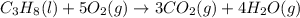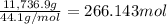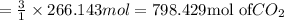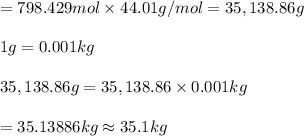What mass of carbon dioxide (in kg) is produced upon the complete combustion of 18.9 l of propane (approximate contents of one 5-gallon tank)? assume that the density of the liquid propane in the tank is 0.621 g/ml. (hint: begin by writing a balanced equation for the combustion reaction.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
What mass of carbon dioxide (in kg) is produced upon the complete combustion of 18.9 l of propane (a...
Questions

Mathematics, 19.02.2021 01:40

Spanish, 19.02.2021 01:40

Biology, 19.02.2021 01:40


History, 19.02.2021 01:40

Biology, 19.02.2021 01:40

History, 19.02.2021 01:40

English, 19.02.2021 01:40


History, 19.02.2021 01:40

Spanish, 19.02.2021 01:40

Mathematics, 19.02.2021 01:40


Mathematics, 19.02.2021 01:40

Physics, 19.02.2021 01:40

History, 19.02.2021 01:40



History, 19.02.2021 01:40