
One solution has a formula c (n) h (2n) o (n) if this material weighs 288 grams, dissolves in weight 90 grams, the solution will have a boiling point of 101.24 ° c. find the formula
the molecules of this substance when determining the kb value of water = 0.512 ° c / m and the atomic weight h = 1, c = 12 and o = 16.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1
You know the right answer?
One solution has a formula c (n) h (2n) o (n) if this material weighs 288 grams, dissolves in weight...
Questions

Chemistry, 24.04.2021 22:30

Social Studies, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30

Social Studies, 24.04.2021 22:30

Advanced Placement (AP), 24.04.2021 22:30


Mathematics, 24.04.2021 22:30



Mathematics, 24.04.2021 22:30

Mathematics, 24.04.2021 22:30


Mathematics, 24.04.2021 22:30

Geography, 24.04.2021 22:30


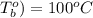 = (100 + 273) K = 323 K,
= (100 + 273) K = 323 K,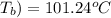 = (101.24 + 273) K = 374.24 K
= (101.24 + 273) K = 374.24 K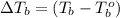

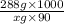
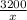
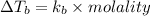
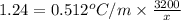
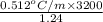
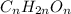 .
.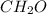 and mass is 30 g/mol. Hence, calculate the value of n as follows.
and mass is 30 g/mol. Hence, calculate the value of n as follows.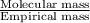
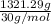
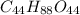 .
.

