
Chemistry, 28.09.2019 01:30 giavanleer14
Avoltaic electrochemical cell consists of a copper electrode in a cu2so4(aq) solution, and a palladium electrode in a pdso4(aq) solution at 25°c. the salt bridge consists of a solution of kcl(aq).
what is the concentration of the cu+if the concentration of the pdso4 is 0.498 m and the measured cell potential is 0.447 v?
given: cu+(aq) + e- ↔ cu(s) e°=+0.521 v
and pd2+(aq) + 2e- ↔ pd(s) e°=+0.987 v

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Avoltaic electrochemical cell consists of a copper electrode in a cu2so4(aq) solution, and a palladi...
Questions




English, 02.12.2019 05:31

Mathematics, 02.12.2019 05:31


Mathematics, 02.12.2019 05:31

English, 02.12.2019 05:31



Mathematics, 02.12.2019 05:31

Mathematics, 02.12.2019 05:31

Mathematics, 02.12.2019 05:31

History, 02.12.2019 05:31


History, 02.12.2019 05:31




Mathematics, 02.12.2019 05:31

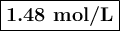
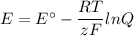
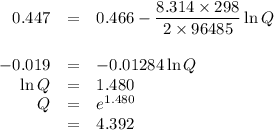
![\begin{array}{rcl}Q & = & \dfrac{\text{[Cu$^{+}$]}^{2}}{\text{[Pd]}}\\\\4.392 & = & \dfrac{{x}^{2}}{0.498}\\\\x^{2}& = & 2.187\\x & = & 1.48\\\end{array}\\\text{The concentration of Cu$^{+}$ is $\large \boxed{\textbf{1.48 mol/L}}$}](/tpl/images/0269/7404/9da92.png)


